ca orbital diagram
This electron configuration shows that the last shell of cadmium has two electrons. Similar to atomic orbitals we can write electron configuration energy diagrams for molecular orbitals Figure 920 Hydrogen molecular orbital electron configuration energy diagram.
Chem Electron Configuration Diagrams Scientific Tutor
83 6 ratings Calcium has atomic number of 20 so it has total of 20 electrons which should.
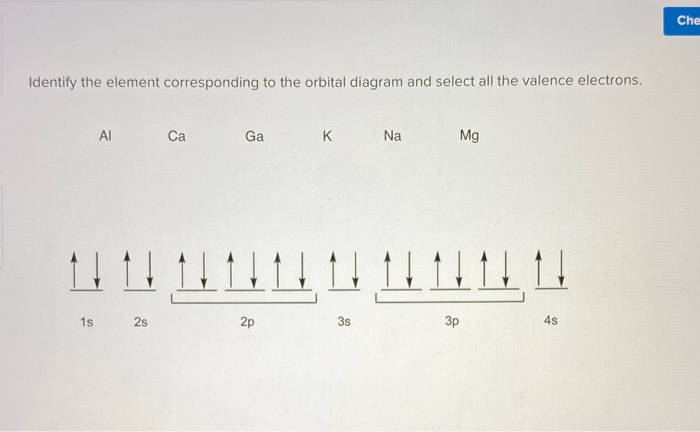
. Calcium Ca Number of electrons per shell 2 8 8 2 Number of valence electrons. An orbital diagram or orbital filling diagram is a type of notation that illustrates an atoms electron distribution and electron spin within orbitals. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2.
Orbital diagrams are known as pictorial descriptions of the electrons in an atom. So the remaining one electron enters the 4p orbital. This is called quantum jump.
Ad Over 27000 video lessons and other resources youre guaranteed to find what you need. Atoms can jump from one orbital to another orbital in an excited state. The orbital diagram is drawn by using.
Below is the electronic. 1s22s22p63s23p64s2 or Ar 4s2. Reduced electronic configuration Ca.
154 gcm 3. Part B Enter an orbital diagram. Since 1s can only hold two electrons the next 2 electrons for Calcium go in the 2s orbital.
In this video we will write the electron configuration for Ca2 the Calcium ion. The Calcium orbital diagram contains 2 electrons in the 1s orbital 2 electrons in the 2s orbital the six electrons in the 2p orbital the two electrons in. Use the Pauli exclusion principle and Hunds rule to work out.
In writing the electron configuration for Calcium the first two electrons will go in the 1s orbital. Get Ca Orbital Diagram MP3 Download 309 MB on Navidbiglarimusic Quick and Easy - NAVID BIGLARI MUSIC How To Write The Orbital Diagram For Calcium Ca 0215 min 320 kbps 309. Electronic configuration of the Calcium atom.
Three rules governing the formation of orbital diagrams are given. To write the orbital diagram of rubidium Rb you have to do the electron configuration of rubidium. Orbital diagrams are like the configuration notation just introduced except with the spins of electrons indicated.
Orbital Diagram for CalciumCa Electron configuration of calcium in the excited state. The orbitals are 1s 2s 2p 3s 3p and 4s. View the full answer.
Therefore the gallium full electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 1. The ground-state electron configuration of cadmium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2. Orbital Diagram of All Elements Diagrams.
Which has been discussed in detail above. 1s is the closest and lowest energy orbital. Well also look at why Calcium forms a 2 ion and how the electron configur.
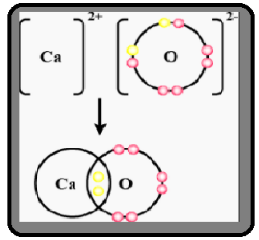
Draw Orbit Structure Diagram Of Calcium Oxide Left Class 12 Chemistry Cbse

Draw An Orbital Diagram And Lewis Structure For A Li And S Homework Study Com

Delocalized Bonding And Molecular Orbitals
Quantum Mechanics
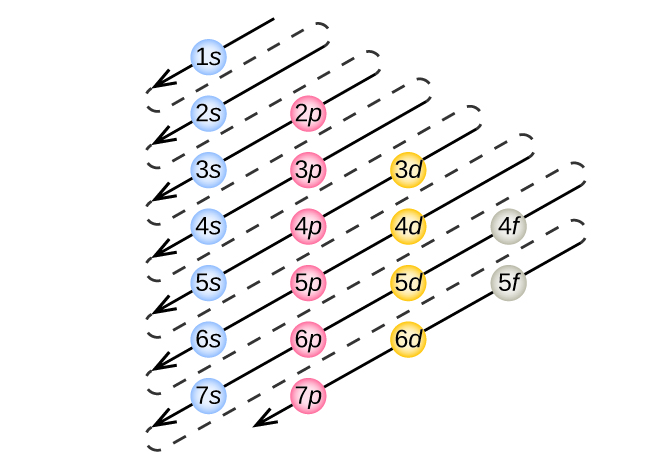
8 5 Electron Configuration In Atoms General Chemistry For Gee Gees

Enlisting Potential Cathode Materials For Rechargeable Ca Batteries Chemistry Of Materials
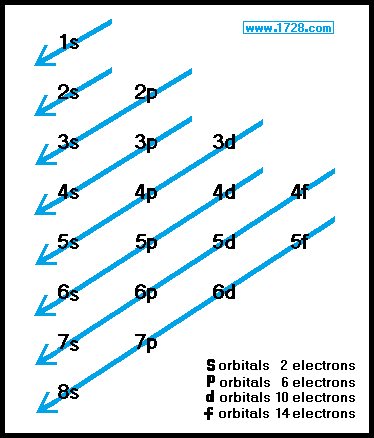
Orbitals And Electron Configuration
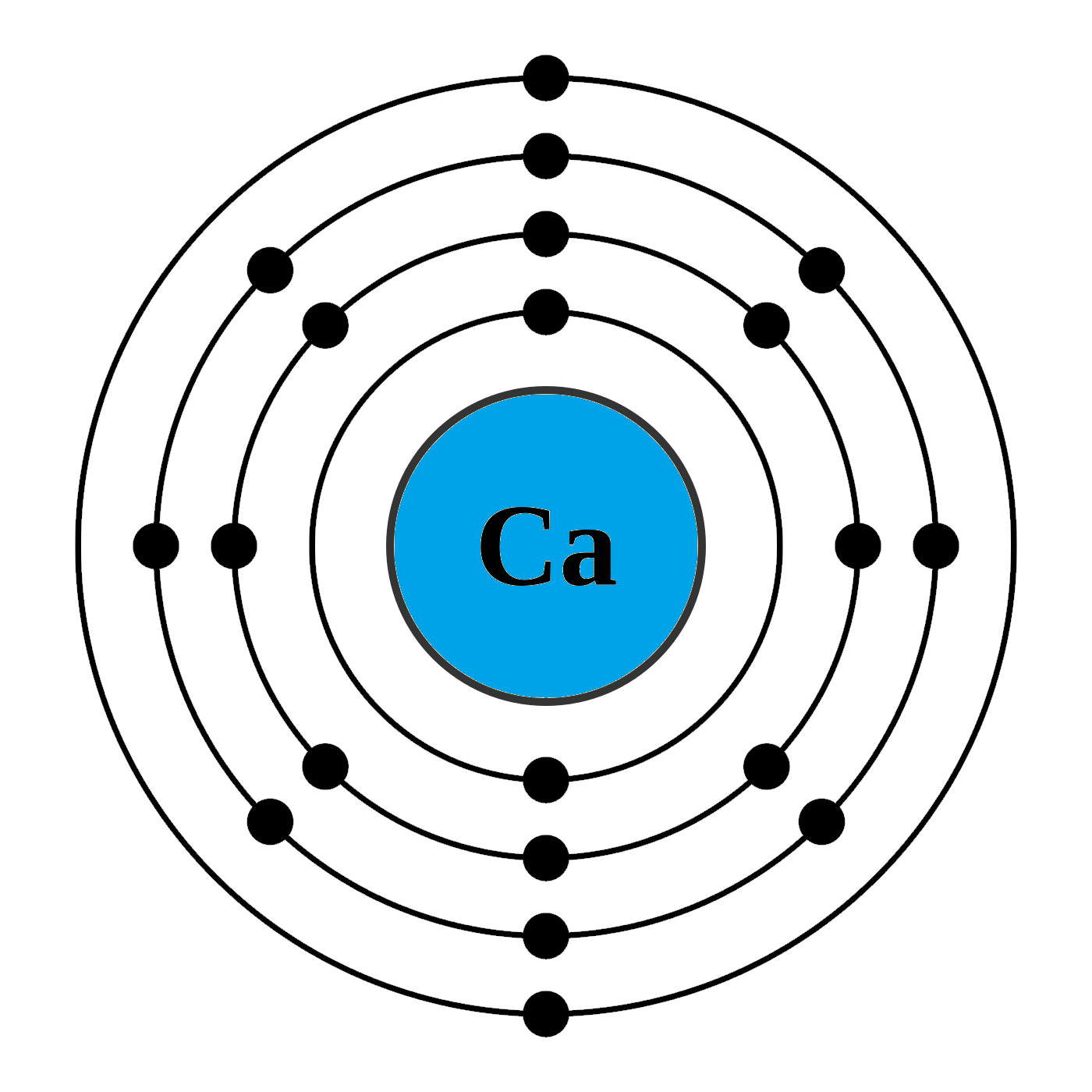
How Can I Draw Electronic Configuration Of Calcium In A Shell

Draw Orbit Structure Diagram Of Calcium Oxide Cao
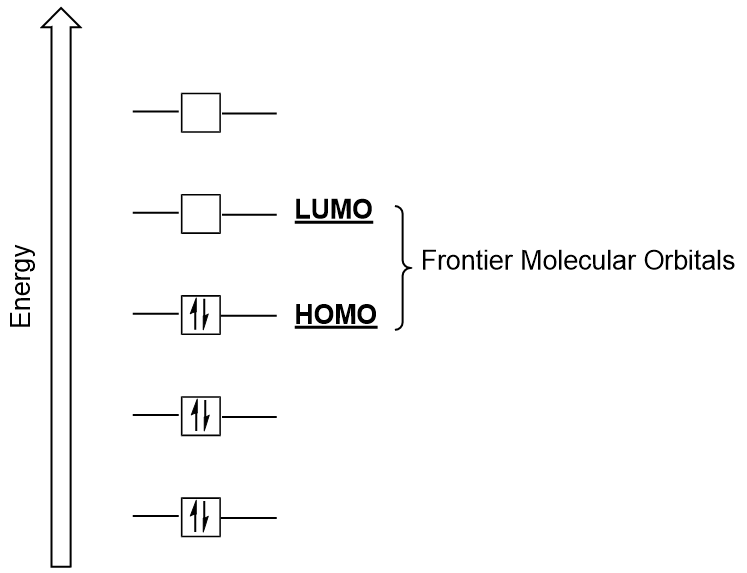
Molecular Orbitals Introductory Chemistry 1st Canadian Edition
The Order Of Filling 3d And 4s Orbitals Chemistry Libretexts

Give Orbital Diagram Of The Following Magnesium Chloride

20 Calcium Quicycle Society

Orbital Notation Watch After Electron Configuration Science Chemistry Showme
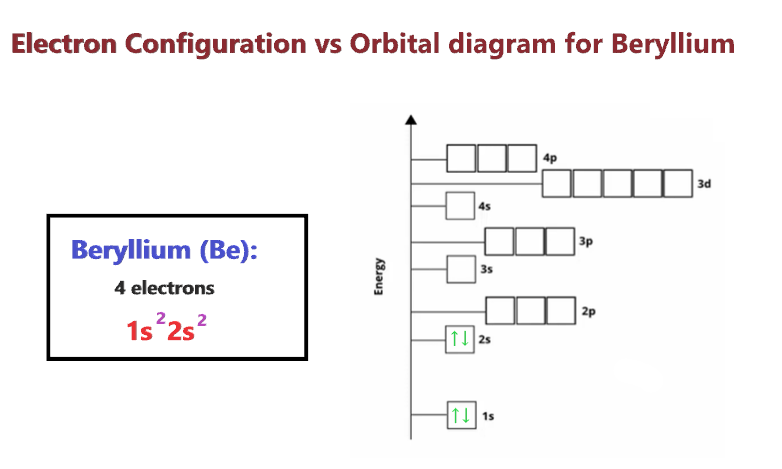
Beryllium Orbital Diagram Electron Configuration And Valence Electrons
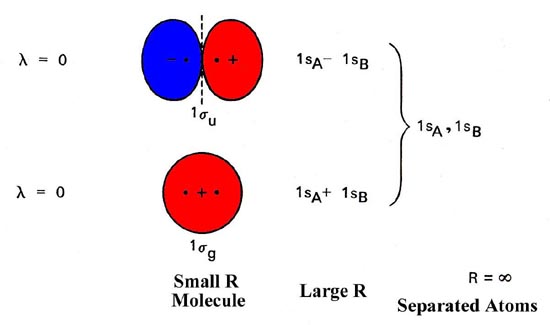
Molecular Orbitals Molecular Orbitals For Homonuclear Diatomics

1 5 Electronic Structure Of Atoms Electron Configurations Inorganic Chemistry For Chemical Engineers